Do you know some SIB brands stated compliance to International Standards Organization does not guarantee safety and efficacy of delivered ventilation when using SIB?
Approximately 10% of newborns require some assistance to begin breathing at birth. Less than 1% require extensive resuscitative measures. 1,2 SIBs are the most common device used to provide positive pressure ventilation during resuscitation at birth world-wide. And the United Nations in 2012 advising the increasing availability of SIBs in resource-constraint countries3,4 because it is a manual device which can be used without the support of external compressed gas source. 1,2
A recent trial shows that the Dispo.Bag Infant Resuscitator proved to be one of the most stable, precise and repeatable life-saving resuscitations among 20 top brands around the world – helping you to reach the highest survival rate during newborn resuscitation.
Compliance of SIB with ISO standards may not guarantee acceptable or safe performance to resuscitate newborn infants.
According to the study, these SIB trials were conducted across 20 models (n = 173) with bench testing to examine relationships between the compression distance of a SIB and delivered volume. There was a scoring methodology described as an innovative, independent and repeatable method of assessment. It examined 173 resuscitators with an average of nine units per model by a computer-controlled two-armed robotic mechanism simulating ‘standard hand compressions’ to SIBs across a precise range of distance. A test lung by Drager was recruited as attached to the SIBs with a respiratory function monitor.
The primary outcome was a "pass"or "fail" to meet the four criteria: minimum average ventilation 5 and 10 mL/kg (2.5 – 5 mL); (Figure 1).

Figure 1 Box and whisker plots of delivered tidal volumes per self-inflating bags model batch at each compression distance with lower regression lines. RU, reusable; SU, single use.
GaleMed Dispo.Bag Infant Resuscitator meets all four criteria to show the quality and performance consistency
The performance of GaleMed Dispo.Bag Infant Resuscitator meets for the precise mean volume consistency (minimum delivered tidal volume) with 7.7% CV match test variance, and 0 high PIP fail (Table 1). There is a total of 67540 compressions from 20 different models provided 48493 compressions with volumes ≥2.5 mL and those compressions with volumes GaleMed SHMC 2203WA SU was not being selected like those 16856 compressions where the SIB failed to deliver a measurable volume when compressed. GaleMed SHMC 2203WA SU and the OEM models that passed on all four characteristics.
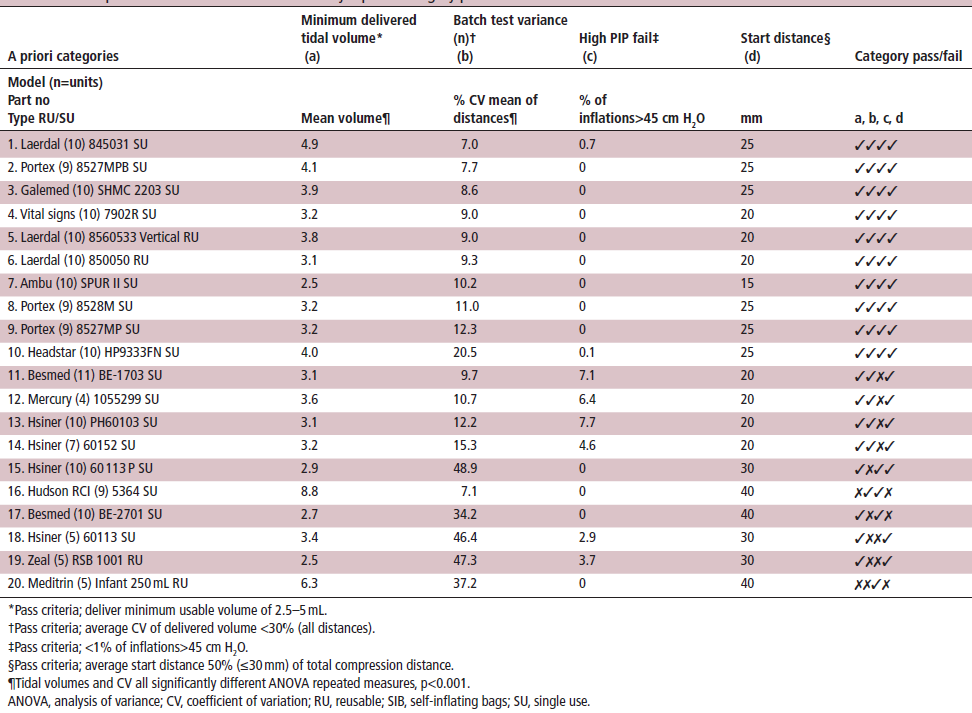
Table 1 Composite scores of Sibs tested sorted by a priori category pass and %CV
GaleMed Dispo.Bag Infant Resuscitator passed the forward air leakage (PIP) test to prove its guarantee the efficacy of delivering a uniform of gas while being compressed.
This research indicates that even though resuscitators compliance with the ISO standards, which do not able to show the safety of infant/pediatric used SIBs (23). The inabilities of the safety pressure limiting valve to prevent PIP in excess of 45cm H2O and the wide CV of intra-batch SIB shows a serious health concern. Another concern is many operator/clinicians could not detect the forward leakage problem routinely during resuscitation (figure 2). The significant forward leakage via the PCV could lead to a complete lack of ventilation reach to the patient, and result in a potential excessive, insufficient or harmful ventilation to the neonatal. 5 The batch variance was performed in this study to show the batch variance of self-inflating bags confirmed that the forward leakage via the PCV could be a treat to the patients. Also, this research examined almost 20 models and found that GaleMed SHMC 2203WA SU and its subsidiaries have a very consistent performance, which is compliance with 0% inflation (Table 1).
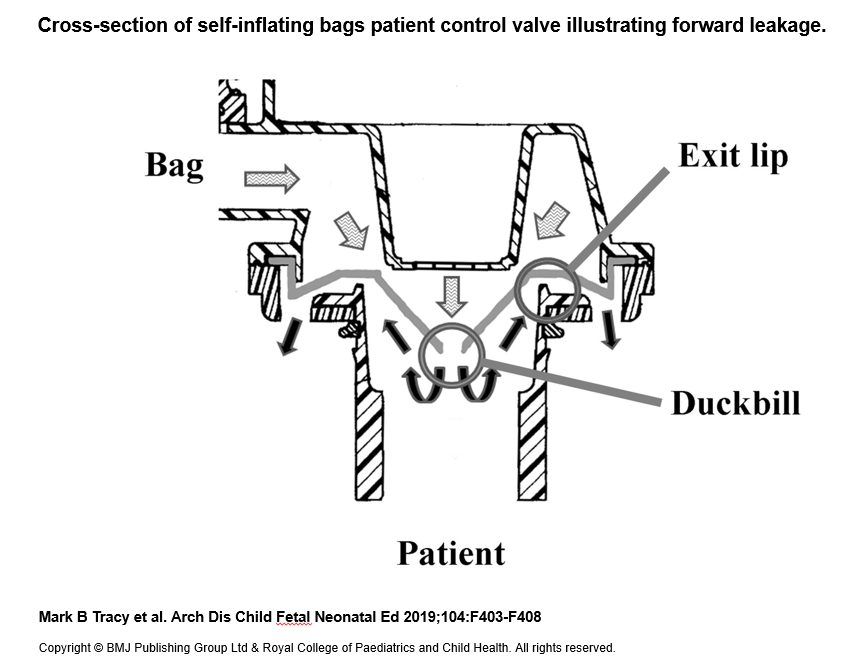
Figure 2 Cross-section of self-inflating bags patient control valve illustrating forward leakage. (Original artwork US Patent 5722394A, modified and illustrated by M Hinder).
The right fit for your facility
The GaleMed dispo.Bag Infant Resuscitator proves it’s an ISO compliance life-saving device that strikes a balance between batch reproducibility and stable performance. It has a much more consistent delivery than almost 20 other brands around the world. The compact design with greater compatible with bacterial filter, pressure manometer and PEEP valve provides the greater usability to make you only require taking your attention off the patients. Proven by international research to be highly performance consistency, this design helps you to optimize your patients’ comfort.
Discover the benefits of the GaleMed dispo.Bag Infant Resuscitator today.
About Author
Tina Chou, Product Specialist
Tina Chou is a product specialist in respiratory care solution at GaleMed. As a Certified Respiratory Therapist,
she
works with product and marketing teams to help develop solutions and analyzing product market trends to improve
product
efficiency. Tina co-works with a R&D team to enhance the product development program at GaleMed.
Note:
This research was conducted by Dr Mark B Tracy, Department
of Paediatrics and Child Health, University of Sydney. GaleMed Corporation did not sponsor any research grant in
the
public, commercial or not-for-profit sectors. GaleMed is clear from the competing interests of the brands being
used in
this study.
For more about this research, see more on BMJ Journals
Appendix:
- Wyckoff MH, Aziz K, Escobedo MB, et al. Part 13: neonatal resuscitation: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care (Reprint). Pediatrics 2015;136(Suppl 2):S196–218.
- Perlman JM, Wyllie J, Kattwinkel J, et al. Part 7: Neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015;132(suppl 1):S204–S41.
- United Nations, 2012. Commission on life-saving commodities for women and children http://www.unfpa.org/publications/un-commission-life-saving-commodities- women-and-children (accessed 22 Mar 2018).
- American Academy of Pediatrics, 2016. Helping babies breathe http://www. helpingbabiesbreathe.org/ (accessed 22 Mar 2018).
- Kain ZN, Berde CB, Benjamin PK, et al. Performance of pediatric resuscitation bags assessed with an infant lung simulator. Anesth Analg 1993;77:261–4.