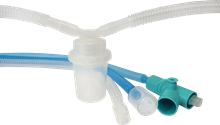
- Upgraded nBag (PEEP valve) and more Babi.Plus™ are under development


Our New Revolution
Our Story & History
Our Innovative Products
Our team of neonatologists, respiratory therapists, nurses, and biomedical engineer s together with over 100 years of experience created Babi.Plus™ from concepts to a real life-saving system